Red and Green Slime Show Properties of Light and More: An Introduction to Slime
The actual science portion of this activity brought to you by the
North Carolina School of Science and Mathematics
Research in Chemistry Class
(Jenifer Brown, Jessie Duan, Alex Li, Nic Liu, Joseph Moo-Young, Sam Stone, and teacher Myra Halpin)
Students delight in late-night comedy sketches showing that viscous substance, snot, endlessly streaming from a character’s nostrils. It’s “gross.” Now you can help your science “phenoms” concoct the slime needed to re-create that charming scene at will.
Highbrow entertainment isn’t the only use for slime. It also has practical applications: a lubricant in eye drops, an alternative to surgery for stopping blood flow to a tumor, an anti-hemorrhaging agent, sizing in fabrics, reinforcement for concrete (in fiber form), and many other medical and industrial uses. You can even use it in your classroom. After the procedures below for creating slime, look for a how-to lesson that uses the oozing mass to teach properties of light, interesting activities for analyzing slime, and an activity for making slime fiber.
What gives slime its unique snotty qualities?
The laugh-trigger for the gross-at-heart enjoys the formal name polyvinyl alcohol (PVA) slime. It is a high viscosity, non-Newtonian fluid with interesting characteristics.
- Pull slime gently and it stretches into a thin film.
- Tug slime quickly and it breaks.
- Hit slime and it doesn’t splash or splatter.
- Stuff slime through a tube and it appears to swell as it exits the tube.
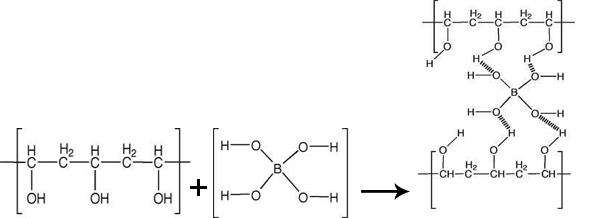
Create PVA slime by mixing a 4% solution of PVA with a solution of sodium borate, known as borax. The molecular bonding of PVA with the borate ions explains the slime-forming process.
 |
|||
| PVA | H4BO4 | Slime | |
Cross-linking occurs when PVA is added to sodium borate; the electronegative oxygen on borate forms weak hydrogen bonds with the hydroxyl groups of PVA. The unique properties of PVA slime are a result of this cross-linking and make slime a useful teaching material. Plus the ingredients are cheap and readily available, PVA slime is nontoxic, and students enjoy snot-like substances.
Create PVA slime in your lab
Materials (small recipe)
- 1.6 g Sodium Borate Decahydrate
- 8 g Polyvinyl Alcohol (PVA), Powder
- 0.02 g Sodium Fluorescein
- 0.004 g Bromphenol Blue
- 240 mL Water
- Stirring Rod
- Glass Container (larger than 40 mL)
- Heatproof Container (larger than 240 mL)
- Microwave Oven or Hot Plate
Procedures
- Dissolve 1.6 g sodium borate decahydrate in 40 mL water. Stir until completely dissolved, approximately 15 min with frequent stirring.
- Dissolve 8 g polyvinyl alcohol (PVA) into 200 mL water. Microwave gently at repeated 30-sec intervals, stirring between intervals until dissolved, approximately 20 min. (An alternative is to use a hot plate to dissolve the PVA into solution, but it takes longer. Purchase a 4% solution if you want to save time.)
- Mix 0.02 g sodium fluorescein and 0.004 g pH indicator bromphenol blue into the sodium borate decahydrate solution.
- Pour the sodium borate decahydrate solution into the 200 mL PVA solution while slowly mixing. Slime should form.
Keep the sodium borate solution and PVA solution in a 1:5 volume ratio if you increase or decrease the amount of slime you make. Below is a picture of slime made with this formula. The slime appears green when flat on a sheet of white paper. Slime appears red as white light passes through it. Make slime more “snot-like” by increasing the PVA to sodium borate ratio.
 Bromphenol blue slime
Bromphenol blue slime
Properties of light
Objects either absorb or reflect incoming light waves. Some light-absorbing substances reemit the light a different color.
In each molecule, electrons vibrate at a natural frequency. When the incoming light-wave frequency exactly matches the natural frequency of an object’s molecules, the light’s energy converts to the electrons’ vibrational energy, which in turn transfers into thermal energy. If all light is reflected, the object appears white. If all light is absorbed, the object appears black.
In slime, fluorescein absorbs the blue-green light and fluoresces to emit green light, while bromphenol blue absorbs every color except red. Thus, when looking at slime from the same side as the light, we only see the slime’s surface fluorescence, which is green. However, when looking from the side opposite the light, the light waves transmitted through the slime also go through the bromphenol blue, where only red light can pass. This shows in spectra below:
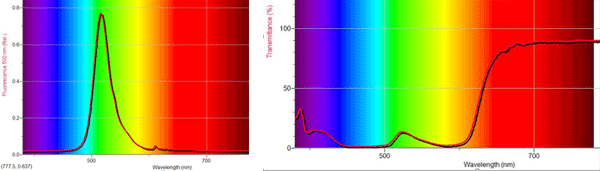 |
|
| Caused by fluorescein | Caused by the bromphenol blue |
| Slime flat on the table absorbs blue-green and fluoresces green. |
Slime held up to the light abosorbs blue-green and transmits red. |
Additional classroom activities
What is color?
Use slime to help students investigate what gives objects their color. Students can vary the amount of bromphenol blue to change the slime’s color from purple to red. Or they can use the above formula but substitute another pH indicator for bromphenol blue.
The following data table shows the color of slime left on a black lab table (Fluoresced Color) and viewed in front of a light (Transmitted Color).
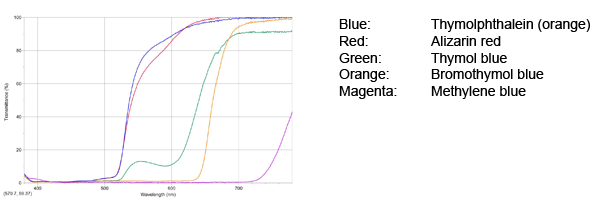
Indicator Fluoresced Color Transmitted Color Bromphenol blue Green Red/purple Methylene blue Green Blue Thymol Blue Green Yellow Alizarin red Yellow Yellow Thymolphthalein (orange) Yellow Yellow If you have a spectroscope, students can investigate the wavelength transmitted and predict the effect on the color of the slime.
Analysis of pH indicators
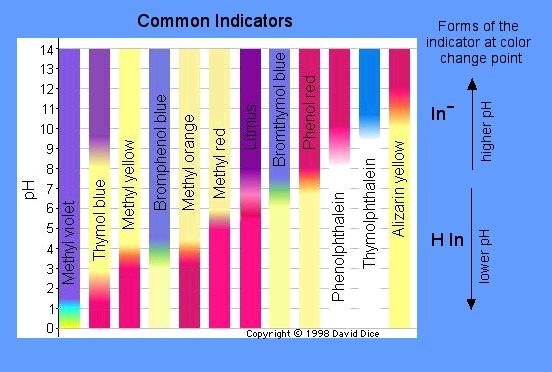
pH indicators change color when the H3O+ concentrations change. This is because adding or removing H3O+ ions causes a change in the chemical structure of indicators. For example, examining the pH indicator bromphenol blue, at pH levels less than 3.0 the acidic solution appears yellow. At pH levels of 4.6 or greater, the solution appears purple. This is demonstrated in the figure below (bromphenol blue is the 4th bar from the left).
Spectrophotometer activity
Before making slimes, students can analyze the dyes of the slimes to predict which dyes will work best. Have students use a spectrometer to measure the transmittances of the 5 dyes. The graphs should look like this.
Is slime a solid or liquid?
Have students observe and describe the behavior of slime.
- Can you pour slime?
- How thin can you stretch slime?
- Hold slime in your hand and quickly pull it. What happens?
- Repeat step c but pull slowly. What happens?
- Try pushing slime through a small tube. Describe what happens.
A liquid fluid (as opposed to a non-Newtonian fluid) takes the shape of its container and is free-flowing. Different fluids have different resistances when deformed by external forces. This is known as viscosity. For example, water pours more easily than honey; thus, honey is relatively more viscous than water. Slime is a non-Newtonian fluid.
Non-Newtonian fluids such as pudding, cornstarch paste (oobleck), and slime don’t pour easily. When you put pressure on slime it will expand, much like wet sand. Slowly stretched, it will thin and flow, but applying pressure quickly breaks slime.
The pH properties of polyvinyl alcohol slime
The pH of polyvinyl alcohol slime is approximately 9.0. This basic slime contains hydrogen bonds that help maintain firmness and adherence. The addition of an acid such as HCl helps break the hydrogen bonds. If hydrogen bonds help keep the polymer together, how will a loss of hydrogen bonds affect the slime’s physical properties? The following activity helps answer that question.
Materials
- 5–7 mL HCl Solution (0.01 M)
- 5–7 mL NaOH Solution (0.01 M)
- Marble-Sized Piece PVA Slime
- Small Beaker or Plastic Cup
- Stirring Rod
- Gloves
- Goggles
- Lab Coat
Procedures
- In your small beaker or plastic cup, add approximately 5–7 mL of 0.01 M HCl to a marble-sized piece of slime.
- Mix the slime and the HCl solution with the stir rod until adequately mixed.
- Pour off excess water and have your students observe the texture change of the slime. Describe your observations.
- Now add the same amount of NaOH solution (a base) to the slime and let it sit for 2–5 min, stirring occasionally.
- Observe and record your observations.
- How does pH affect the firmness of slime?
After adding HCI After adding NaOH
Polyvinyl alcohol fibers activity
Materials
- 4% PVA Solution
- 50-mL Beaker
- 10 mL Acetone
- Forceps
- Foil or Wax Paper
Procedures
- To make polyvinyl fibers, first make a 4% polyvinyl alcohol solution.
- Pour 10 mL of the solution into a 50-mL beaker.
- Tilt the beaker slightly and pour in (down the side) 10 mL of acetone. Do not let the solutions mix. This is important, otherwise, a large clump will form in the beaker.
- Use forceps to pull the film towards the top of the beaker to form a fiber. If the end of the fiber breaks, dip it back into the solution and then pull it up again.
- Lay the fiber on foil or wax paper to dry.
Unstretched Stretched When dry, the fiber is hard and brittle Dipped in water, the fiber becomes
flexible and stretchless again.
Conclusion
Now that your students know the chemistry of slime, see what creative uses they find for the product of this lab.