Making Magnetic Slime
 Crystal Risko
Crystal Risko
Product Developer
Oozy, gooey, sticky, and fun, the creation of magnetic slime is a good way to get your students interested in polymers and magnetism. In this lab activity students will create a poly(vinyl alcohol) polymer that has iron(II, III) oxide incorporated into its matrix. Because of the incorporated iron(II, III) oxide, the slime is attracted to neodymium magnets.
Slime
Scientifically speaking, slime is a colloid, a type of homogeneous mixture in which 1 state of matter is integrated within another. Slime contains liquid water integrated into a solid network of cross-linked polymer chains, forming a gel. Like a solid, slime can be formed into a ball and sustain its shape (if only for a limited time). However, slime contains over 90% water and can flow like a liquid. The interactions that bind the solid and liquid parts of slime are based on the structure of its components—poly(vinyl alcohol), borax, and water. Each of these components has unique and interesting properties.
Poly(vinyl alcohol), also known as PVA, is a polymer used to make plastics, such as chemical-resistant films, surface coatings, and molding compounds. The term polymer comes from the Greek poly, meaning “many,” and meros, meaning “part.” A polymer is a large molecule made up of many smaller molecules, called monomers. In Greek, mono means “one.” As the name suggests, poly(vinyl alcohol) is made of the monomer vinyl alcohol.
Borax is the common name for sodium tetraborate decahydrate, Na2B4O7 • 10H2O, also called sodium borate. Borax occurs naturally and is used in laundry and cleaning products. It is a mineral sediment formed after repeated evaporation of seasonal lakes. Borax is the salt of a strong base and a weak acid. When added to water, borax forms the borate ion.
When PVA and borax are mixed, the borax connects chains of PVA in such a way that a 3-dimensional network of chains is formed (Fig. 1).
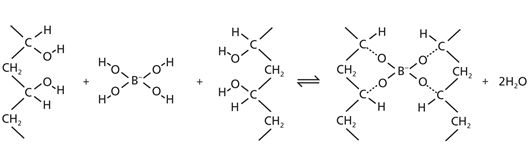
Figure 1 Slime polymerization.
Some of both the water molecules that are produced and the water molecules that are already present are trapped in the polymer cross-links. This is due to electrostatic attraction (positive to negative) between the PVA/borate polymer and the water.
Magnetism
Magnetism is the ability of a material to attract iron and produce a magnetic field. The word magnet comes from the Greek word, magnes, meaning “the stone of Magnesia.” In the region known as Magnesia, the Greeks found a magnetic iron ore called lodestone that attracted other iron-containing materials. Magnetism does occur naturally, as the Greeks discovered, but it also can be created or “induced” in certain materials, especially iron, nickel, and cobalt. In many instances, this magnetic property is weak or temporary. Whether natural or induced, permanent or temporary, each magnet produces a magnetic field. A magnetic field is an area where magnetic lines of force can be felt or measured. These lines of force seem to “flow” through and around the magnet, out from the north pole, then back in through the south pole of the magnet (Fig. 2).
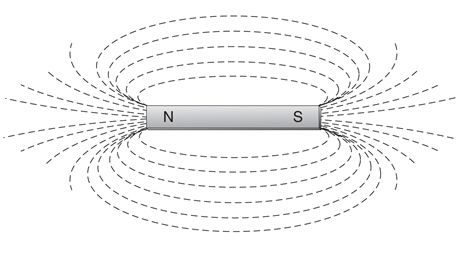
Figure 2 Magnetic lines of force.
Scientists use the orientation of magnetic domains to help explain the behavior of magnets. A magnetic domain is a molecule of a substance that acts as a tiny magnet and exhibits a tiny magnetic field. In non-magnetic objects, the domains are oriented randomly (Fig. 3 left). As a result, the magnetic fields cancel each other out. In magnetic objects, however, most or all of the domains are oriented in the same direction; they are aligned so that their magnetic fields combine to create a net magnetic field (Fig. 3 right).
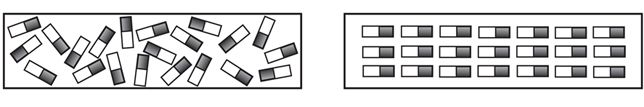
Figure 3 Unmagnetized material (left) and magnetized material (right).
Materials (per group)
- Sodium Borate (borax), 0.2 g (item #888560)
- Polyvinyl Alcohol (PVA), 4%, 40 mL (item #882372)
- Iron(II, III) Oxide, 5 g (item #862630)
- Neodymium Magnets (item #758310A)
- Large Weigh Boat (item #702334)
- Water, 15 mL
- 2 Spoons
- Balance
- Graduated Cylinder, 10 mL
- Graduated Cylinder, 50 mL
- 2 Plastic Cups (100 mL or larger)
- Small Sealable Plastic Bag
- Soap and Water for Washing Hands
Procedure
- Place a neodymium magnet in a small plastic bag. This will keep it clean while you perform your experiments.
- Place 15 mL of water in 1 of the 2 plastic cups.
- Add 0.2 g of borax to the water. Stir the solution with a spoon until all of the borax has dissolved.
- In a separate cup, place 40 mL of PVA.
- Add 5 g of Iron(II, III) oxide to the PVA. Stir the mixture with a clean spoon until the Iron(II, III) oxide is dispersed evenly.
- Pour the borax solution into the PVA mixture.
- Stir the mixture with a spoon. The solution should come together quickly in a gel, forming slime.
- Pick up the slime and mix it with your hands, then place it in a large weigh boat.
- Bring the magnet in the plastic bag towards the slime but do not touch it. Determine how far from the slime the bag is when the slime starts being pulled toward the magnet.
- Remove any slime from the exterior of the plastic bag and from your hands by washing them with soap and water.
- Open the plastic bag and add another magnet.
- Bring the magnets in the plastic bag toward the slime but do not touch the slime. Determine how far from the slime the bag is when the slime starts being pulled towards the magnets.
- Repeat steps 10–12.
Related products
- Carolina ChemKits®: Slime Time (item #840808)
- Rainbow of Slime Class Chemistry Kit (item #840800)
- Cosmic Slime Class Chemistry Kit (item #840804)
- Discovering Polymers Demo Chemistry Kit (item #840475)
- Introduction to Magnetism Kit (item #758231)
- Carolina™ Introduction to Electromagnetism Kit (item #758661)

