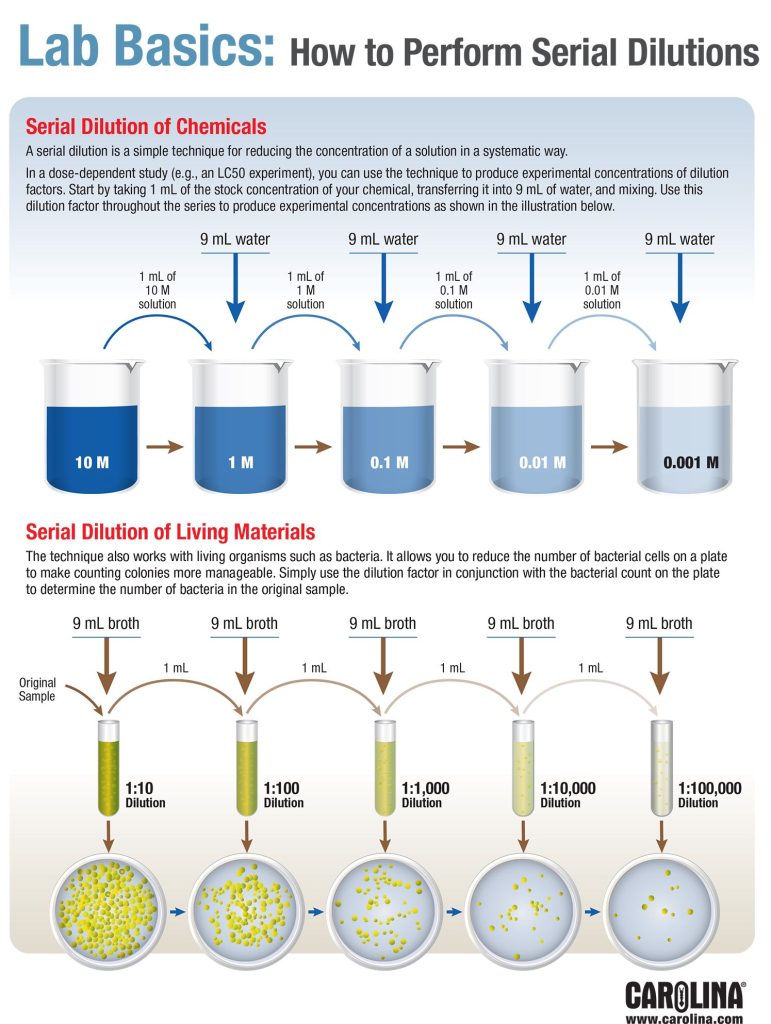
A serial dilution is a simple technique for reducing the concentration of a solution in a systematic way. In this activity, students prepare beverage solutions over a wide range of concentrations and determine the minimum concentration required to taste the sweetness of a solution.
Safety
This activity involves taste-testing a prepared solution. Note: Do not use laboratory materials or glassware. All materials should be purchased for consumer use and discarded at the conclusion of the activity. Consider performing the One in a Million activity in the school cafeteria or trading classrooms with a food science teacher for the day. Emphasize that students should not eat, drink, or chew gum during activities performed in their science classroom.
Materials (per student)
- Presweetened Powdered Drink Mix (Kool-Aid® or similar)*, 8 to 9 g
- Plastic Spoon
- Large Cup (10 oz or larger)
- 6 Small Cups (4 oz or larger)
- Permanent Marker
- Tap Water
*Or you can add table sugar to unsweetened drink mix according to instructions printed on the package. Do not add water. Students will add water during the activity.
Student procedure
- Use a permanent marker to label the 6 small cups with letters A through F, respectively.
- Use a permanent marker to label the large cup “water.”
- Fill the large cup with tap water.
- To prepare solution A:
- Add 1 level spoonful of presweetened powdered drink mix to the small cup labeled “A.”
- Use the plastic spoon to transfer 9 spoonfuls of water from the “water” cup to the cup labeled “A.”
- Use the plastic spoon to stir the contents of the cup until they are completely dissolved.
- To prepare solution B:
- Add 1 level spoonful of solution A to the cup labeled “B,” and then rinse off the spoon.
- Use the plastic spoon to transfer 9 spoonfuls of water from the “water” cup to the cup labeled “B.”
- Use the plastic spoon to stir the contents of the cup until they are completely dissolved.
- Prepare solutions in the remaining cups by repeating step 5 using
- Solution B and the cup labeled “C.”
- Solution C and the cup labeled “D.”
- Solution D and the cup labeled “E.”
- Solution E and the cup labeled “F.”
- Record observations about the intensity of the color in each cup.
- Beginning with cup F, taste-test each solution by sipping a small amount of liquid from each cup. Note when you can first discern the sweet taste of the solution in the cup.

Extension activities
- Have students determine the concentration of each cup in terms of (volume of solute) / (total volume). Next, ask students to determine which cup represents 1 part per million (ppm), and how many more cups would be required to represent 1 part per billion (ppb).
- Measure the mass of a level spoonful of sweetened powdered drink mix. Next, measure the mass of a spoonful of water. Have students determine the concentration of each cup in terms of (mass of solute) / (mass of solution).
Related products
- Inquiries in Science®: Testing Water Pollution Kit (item #251415)


1 comment
I appreciate the lessons and the materials kits.