My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
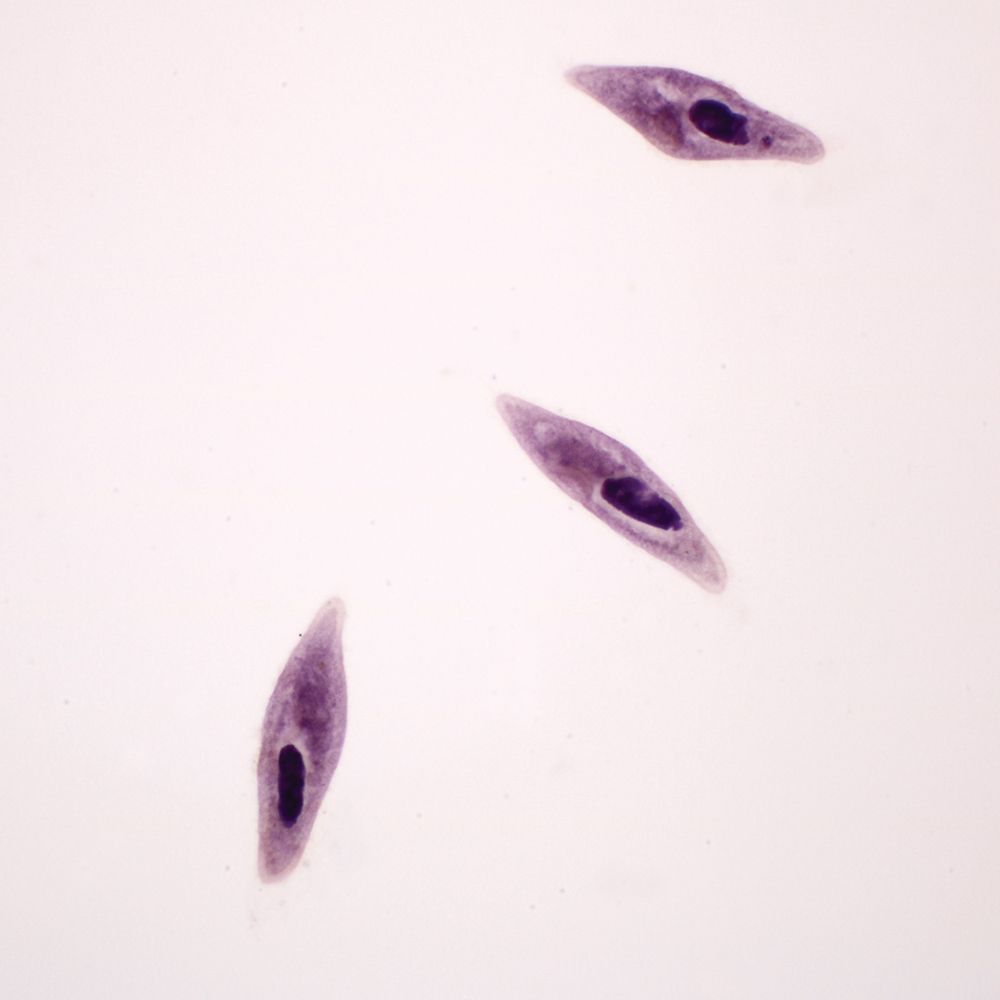
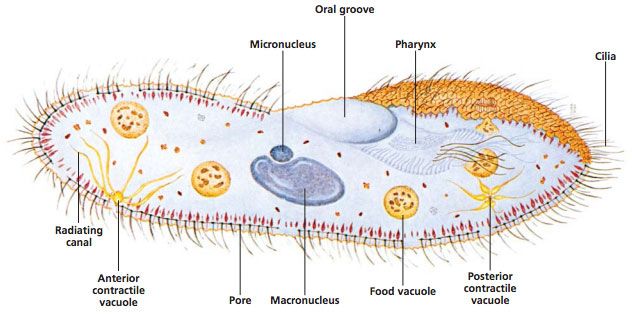
In this lab, students observe Paramecium as an example of a ciliated protist. Ciliate cells are often structurally complex. Traditionally, Paramecium was classified under Domain Eukarya, Kingdom Protista, and Phylum Ciliophora. However, it is widely recognized that Protista is not a natural grouping. Currently ciliates are often grouped with dinoflagellates and sporozoans in the clade Alveolates. Today, “protist” is best used to denote a grade of organization including eukaryotic organisms that are unicellular or multicellular without tissues.
Paramecium culture (131540, 131554, or 131560) for up to 30 students or 60 students working in pairs
Some prefer 131542 Vitachrome® prestained Paramecium. A stained microscope slide (296962) will show more clearly the macro and micro nuclei and other features. If the paramecia swim too rapidly, they can be slowed down with Protoslo® (885141). To use Protoslo, thoroughly mix a drop of Protoslo with the drops of culture on a slide before adding the cover glass. The drops of Protoslo and culture must be well mixed or the paramecia may be forced to the margins when the coverslip is added.
Ensure that students understand and adhere to safe laboratory practices when performing any activity in the classroom or lab. Demonstrate the protocol for correctly using the instruments and materials necessary to complete the activities, and emphasize the importance of proper usage. Use personal protective equipment such as safety glasses or goggles, gloves, and aprons when appropriate. Model proper laboratory safety practices for your students and require them to adhere to all laboratory safety rules. Paramecia are not parasitic or pathogenic. Even so, know and follow your district’s guidelines so you are prepared if a student should ingest a culture. Cultures remaining after the completion of the activities can be flushed down a sink with tap water. The chlorine and chloramines in most tap water will kill the paramecia. If your tap water is not chlorinated, pipet 1 mL of bleach (sodium hypochlorite) or isopropanol (rubbing alcohol) into the culture and wait 15 minutes before flushing down the sink.
Students can work individually or in groups of two.
When you receive your Paramecium culture, remove it from the shipping container. Loosen and remove the lid. Use the dropping pipet included with your order to aerate the culture. After aeration, replace the lid loosely on the jar but do not screw it down. Leave the culture undisturbed for 5 to 15 minutes and then examine it with a dissecting scope (stereomicroscope) at 20 to 40x. Bacteria are decomposing the wheat seeds in the bottom of the culture. You will notice fuzzy areas on the bottom of the jar where bacteria are concentrated. Paramecia feed on the bacteria, so they will also be concentrated in these areas. Have students take samples from these areas of concentration.
Set up workstations for each Paramecium culture. Each station should have the following:
Occasionally, a student may make a slide that does not contain paramecia. This is usually due to improper sampling. If students squirt water from the pipet back into the culture jar or use the pipet to stir the culture, this disperses the paramecia and makes them harder to find. If the student squeezes the pipet bulb while the tip is in the culture, this sweeps paramecia away from the pipet tip.
Failure to handle slides or coverslips by their edges can result in transfer of soap residue to the wet mount. Suspect this if you see paramecia that have stopped moving or are misshapen. Rupture of the cell (lysis) may occur. If this becomes a problem, demonstrate again the correct way to handle slides and coverslips by their edges. Some students may need to rinse their hands under running water and dry with plain paper towels before continuing.
Optional:
Paramecia will usually discharge trichocysts when exposed to an acid. Put a drop of vinegar on a drop of culture, add a coverslip, and observe. Some nuclear stains also cause the discharge of trichocysts, so you may be able to combine these two activities. If you have a general nuclear stain (e.g., toluidine blue, methylene blue,), your students can view the macronucleus. Put a drop of culture on a slide and add a drop of stain. The macronucleus stains darker than the surrounding cytoplasm. Staining in this way may distort the macronucleus and the Paramecium as a whole. Try it yourself to see if you are satisfied with the results before deciding to do it as a class activity. Slides prepared by technicians using more advanced methods will show macronuclei much better and will show the micronuclei as well.
Item 131558 consists of separate cultures of mating types of Paramecium multimicronucleatum for observing conjugation. Paramecium bursaria, item 131548, has zoochlorellae as symbionts and can be used as a model of how chloroplasts might have arisen through endosymbiosis. Students can observe other ciliates to better appreciate the wide variety of organisms in this group. Euplotes (131480) has its cilia banded together in tufts that it sometimes uses to creep along surfaces. Spirostomum (131590) is one of the largest ciliates and has an elongated worm-like shape. Stentor (131598) is a spectacular, large ciliate that is pigmented, which is unusual for a protozoan. Using books and the Internet, students can report on what is known about the functions of the parts of the Paramecium that they have observed.
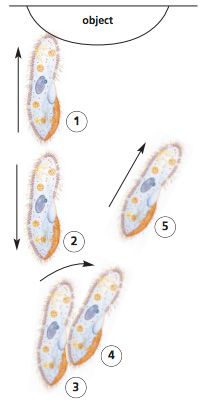
Follow a paramecium as it swims. You may have to switch to low power for this. Do you see anything that indicates that the Paramecium has an anterior end? Explain
Yes, it swims with the more rounded end forward unless it contacts something and reverses.
Draw a diagram showing the pattern of movement that you observed as a Paramecium swam.
It can be difficult to reconstruct the full range of motion that a Paramecium makes when swimming. It tends to track a slow spiral through the water, rotating counterclockwise when viewed from the rear.

What happens when a Paramecium meets an obstacle? Include a diagram.
A Paramecium reverses and then veers around the object.
Make a drawing of a Paramecium, labeling the parts.