My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
By Shuana Jordan
Product Developer
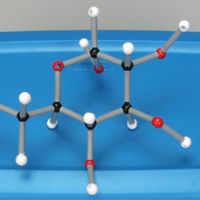
To balance chemical equations, students must know how to count atoms, understand the difference between coefficients and subscripts, and identify reactants and products. Balancing equations reinforces understanding of the law of conservation of mass as students keep in mind that the same number and types of atoms must be on the reactant and product sides of the chemical equation.
In this activity, students build models of compounds to help them visualize the reactants and products in chemical reactions and determine the coefficients in chemical equations. This activity is appropriate for a class of high school students working in pairs.
This activity is appropriate for high school students and addresses the following National Science Education Standards for grades 9–12:
| __N2 + __H2 → __NH3 | ||
| Element Symbol< and Gumdrop Color | Reactant Side (final number) | Product Side (final number) |
| N Color: ______________ | ||
| H Color: ______________ | ||
Figure 1 Example data table.
N2 + 3H2 → 2NH3
2Fe + 6HCl → 3H2 + 2FeCl3
CH4 + 2O2 → CO2 + 2H2O
2K + 2H2O → 2KOH + H2
HCl + NaOH → NaCl + H2O
FeS + 2HCl → H2S + FeCl2
C2H4 + 3O2 → 2CO2 + 2H2O
This straightforward activity should clear up any confusion that some students may still have between coefficients and subscripts in chemical equations.