My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
Mike Isley
Product Developer

One of the most popular teacher demos to illustrate the power of atmospheric pressure is to heat to a boil a small amount of water in an aluminum beverage can, then quickly submerge its mouth in an ice water bath. The walls of the can suddenly implode because the atmospheric pressure is greater than the can’s interior pressure. Students can do this activity as a quantitative guided-inquiry exercise to show the relationship between temperature, pressure, and volume of gases.1
Atmospheric pressure at sea level is 101.3 kilopascals, 760 mm mercury, or 14.7 lb per square inch. This would be the amount of pressure exerted on the outside of any empty beverage can. Since the can is open, the air pressure on the inside of this can is the same as the outside pressure. Therefore, the equilibrium of pressures enables the can to maintain its shape. When a small amount of water (20 mL) is added to the can and brought to a boil with a hot plate or Bunsen burner, the water changes into water vapor, driving out all of the air in the can and occupying all of the inside volume. When the can is quickly inverted and placed mouth down in an ice water bath, the water vapor molecules slow down and condense back into liquid water. As a result, the gas volume and pressure inside drops suddenly, creating a partial vacuum. With the drop in pressure inside the can and the atmospheric pressure remaining constant outside it, the imbalance of pressures causes the walls of the can to dramatically collapse.
The activities that follow can be done as demonstrations or in lab groups of 2 to 4 students.
For teacher demonstration or each lab group of 2 to 4 students
Use 3 additional empty aluminum cans for each of the activities that follow.

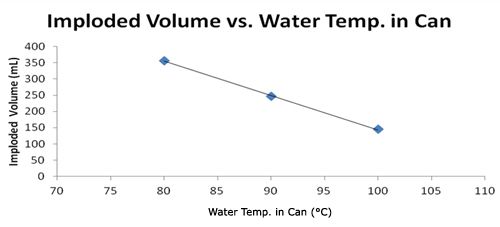
| Water Temp. in Can (°C) | Imploded Volume (mL) |
|---|---|
| 100 | 145 |
| 90 | 246 |
| 80 | 357 |
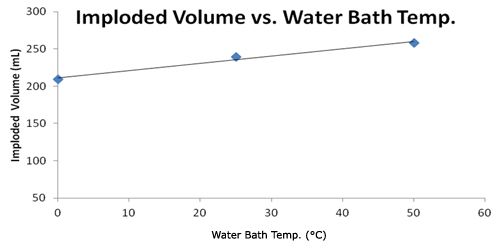
| Water Bath Temp. (°C) | Imploded Volume (mL) |
|---|---|
| 0 | 209 |
| 25 | 239 |
| 50 | 258 |
1Eichler, J. 2009. Imploding Soda Cans: From Demonstration to Guided-Inquiry Laboratory Activity. Journal of Chemical Education 86 (4): 472–474.
2Coke Can Implosion. Available at: http://wn.com/coke_can_implosion Accessed January 8, 2014.