My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
By Polly Dornette
Product Manager
A growing number of people are producing their own biodiesel fuel by transesterification of various triglycerides. With pretzel sticks, marshmallows, and gumdrops, your students can model the steps of this chemical process. This activity, based on one developed by the Educational Division of the North Carolina Zoological Park, can serve to introduce a biodiesel wet-lab activity, to reinforce the chemistry afterward, or it can be presented independently.
Biodiesel is among many potential supplements or replacements for fossil fuel. Some benefits of biodiesel are that it is nontoxic, nonflammable, biodegradable, and it can be produced from waste materials. Although biodiesel can be made from a wide variety of vegetable oils and animal fats, currently more than 90% of the biodiesel produced in the US is from soybean oil. The starting material for production of biodiesel is called the feedstock. An efficient biodiesel feedstock provides a good source of triglycerides without a lot of free fatty acids.
In transesterification, the 3 fatty acid chains (esters) are separated from a triglyceride molecule and bound to the methyl groups from 3 methanol molecules in the presence of a catalyst, producing 3 methyl esters (the biodiesel). The hydroxyl (OH) groups from the methanol replace the esters removed from the triglyceride, producing glycerol, a by-product of biodiesel production.
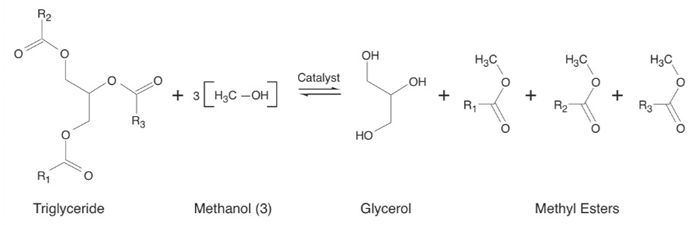
This activity is appropriate for high school students working independently or in small groups and can be completed in 15 min. Depending on the treatment and context, it might also be used with middle school students.
Physical Science
Grades 5–8
Grades 9–12
Science and Technology
Grades 5–12
Science in Personal and Social Perspectives
Grades 9–12
Students should not eat in the science laboratory. If you want your students to be able to eat their models at the conclusion of the lab, conduct the activity outside the laboratory.
Write the transesterification reaction on the board, in the form that best fits the ability level of your students and your learning objective. Distribute the materials and provide students the following instruction: