My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
By Valerie Chase, PhD
Staff Biologist, National Aquarium in Baltimore
501 E. Pratt Street, Baltimore, MD 21202
No other human-caused global change has occurred so abruptly as the increased nitrogen content of our natural ecosystems—not increased carbon dioxide, not increased human population, not deforestation. That the first edition of Issues in Ecology, a new publication from the Ecological Society of America, focuses on how people have altered the global nitrogen cycle indicates the importance professional ecologists place on this problem.
Some of the earliest public attention to the issue appeared in agricultural publications, which addressed the reproductive problems of cows and sheep associated with nitrates in water supplies; this was followed by attention to human health concerns.
Marine and estuarine ecologists weighed in next, demonstrating the role of nitrogen in the overproduction of algae and the changes in algal species in coastal and bay waters "enriched" with nitrogen. In turn, the death and decomposition of algae have led to low oxygen levels (anoxia), which causes mortality in shellfish and fish.
Ecologists have noted the role of nitrous oxides in acid rain and photochemical smog. More recently, they have discovered reduced biodiversity of species in terrestrial ecosystems that are heavily fertilized.
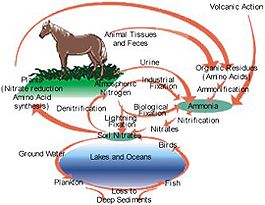
The nitrogen cycle is based on natural events in which atmospheric nitrogen is fixed into compounds, largely as ammonium and nitrate, which are essential mineral nutrients for the growth, repair, and reproduction of plants and algae. Nitrogen enters the food chain through plants, where it appears in a variety of compounds like amino acids. Then it is converted back to atmospheric nitrogen by denitrifying bacteria that live in anaerobic (oxygen-free) mud, or it is returned to the soil as a mineral nutrient by decomposers (Fig.1).
Nitrogen is fixed in a number of natural processes. Some algae and bacteria convert atmospheric nitrogen into plant-useable nitrogen. The best known of these live in a symbiotic relationship with legume roots. Lightning is a much smaller source of fixed nitrogen. Estimates of the annual natural nitrogen fixation prior to the widespread planting of legume crops range from 90 to 140 teragrams (Tg). (A teragram is 1 trillion grams or 1 million metric tons.)
Since nitrogen is often the mineral nutrient limiting crop plant growth, humans have expended great energy increasing its production in plant-usable form. One method is the widespread cultivation of legume crops and forage, like soybeans, clover, and alfalfa. More recently, the manufacture of inorganic nitrogen fertilizer has skyrocketed to double the amount fixed by nitrogen-fixing crops. These 2 sources add approximately 120 Tg of nitrogen per year.
Burning forests, grasslands, and fossil fuels releases nitric oxides and other gases. The high-temperature combustion also oxidizes some of the atmospheric nitrogen. Nitrous and nitric oxide gases add the insult of forming acid rain and photochemical smog.
Even remote regions of the world receive some nitrogen enrichment from atmospheric pollution, but because inorganic fertilizer and legume crops, cars, factories, and power plants are not evenly distributed across the Earth, some regions are more affected by nitrogen release than others. Since nitrate, ammonium, nitric oxide, and nitrous oxide dissolve in water, all end up eventually in groundwater and in aquatic ecosystems (Fig. 2). The water in developed or highly agricultural regions generally has the highest levels. Denitrifying bacteria living in anoxic places (like the mud in wetlands) are the only means by which fixed nitrogen naturally returns to the atmosphere as an inert gas.
Nitrogen fixed in plant products is eaten by animals and often returns to natural systems in the form of sewage or manure, and some of the nitrogen is recycled into crops. Unless the amount and timing of the manure or sludge spreading are carefully monitored, much of the nitrogen from these sources also enters streams, rivers, bays, or coastal seas. Large-scale aquaculture using inorganic fertilizer can also produce severe nutrient loading.

Nitrate in drinking water can be dangerous for young animals, including humans. In oxygen-poor stomach environments nitrate is reduced to nitrite. Most adult humans convert only about 5% to nitrite, but babies have less stomach acid, a condition that favors nitrite production.
Fetuses and infants have a different form of hemoglobin from children and adults. Nitrite combines irreversibly with fetal hemoglobin, preventing it from carrying oxygen. For this reason the US Environmental Protection Agency (EPA) sets a limit of 10 parts per million (ppm) of nitrogen as nitrate for public drinking water. There is no evidence that babies have died in the United States from nitrate pollution, but in Europe, where levels of 2,000 ppm have been measured, some have. Miscarriages and brain damage are also possible.
American farm animals have been affected, however. Ruminants like sheep and cows, with their elaborate, highly anoxic stomachs convert nitrate in water and nitrogen-rich feed stock to nitrite. Reproductive failure in cows and sheep has been traced to high nitrate ingestion on farms where wells have tested as high as 700 ppm of nitrogen as nitrate.
Fertilization can change the relative competitive relationships among species, favoring a few at the expense of many others. Experiments on a Minnesota native grassland measured species diversity from 20 to 30 species per square meter; this was reduced to dominance by a single introduced grass after a number of years of fertilization. Changes in species composition can occur in enriched aquatic systems, also, although typically nitrogen is more likely to be the growth-limiting nutrient in estuarine and marine systems while phosphate is the growth limiter in freshwater environments.
Nutrient enrichment is implicated in red tide episodes and in recent Pfiesteria events in North Carolina and Maryland (suspected to be precipitated by phosphate in hog and chicken manure runoff). In both cases, naturally occurring toxic dinoflagellates incurred huge population growth over a short period of time, causing fish kills. A spring 1996 red tide in Florida also produced manatee deaths from inhalation of toxic compounds.
Toxins from Pfiesteria produce neurological problems in humans, and the organism itself attacks fish and human flesh. Thus, the impact of nutrient loading may range from a loss of biodiversity to fish kills and human health hazards.
Nitrate enrichment can alter an entire estuary. Phytoplankton growth is so dense in the summer in the Chesapeake Bay that light penetrates less than 0.5 m. Sea grasses rooted in the bottom have insufficient light for growth. As the phytoplankton sink to the bottom, they are decomposed by aerobic bacteria, which can completely deplete the oxygen in bottom waters during calm weather. Deeper waters of the Bay experience anoxic events each summer. Sea grasses have declined in many areas, also, mainly because of nutrient loading.
Just as the nature of the problem—sewage, fertilizers, manure, or power plants—varies with the region, so does the mix of actions to address these problems. Nitrogen that enters systems from a specific source—such as a smokestack or a sewage outfall—can be addressed with regulations and technological solutions.
Most of the nitrogen sources are not within the current scope of regulation, however. No one measures or regulates how much fertilizer homeowners, farmers, or golf courses use. Although some sources are very hard to regulate, we might curtail fertilizer use through education, incentives, and taxes.
Because nitrate in water is easily and safely measured and because its presence is a widespread problem, it makes an excellent topic for class projects in ecology, environmental science, or agriculture. During the summer of 1990, I started an environmental science course for secondary teachers. Over a 2-week period we worked our way across Maryland from the mountains to the sea, examining a different environmental issue each day. The first mucky, algae-ridden pond in the mountains barely registered a trace of nitrate (though we did find a stream with a pH of 4.3).
Then we came down out of the mountains. The first stop in dairy country produced 8 ppm of nitrogen as nitrate from a tap at a fast-food restaurant. All across central Maryland we found 6 to 9 ppm in drinking water and in streams and rivers during that dry summer. Algae and aquatic plants can reduce nutrient levels in ponds, lakes, and bays, but groundwater and runoff record the story of nitrate pollution.

From that class on, we have tested nitrate in water. Over the years, the highest nitrate measured in teacher courses was 15 ppm from a well in a very expensive residential area. Ironically, that teacher brought her own well water to class to avoid drinking "nasty" Baltimore tap water, which measured only 3 ppm at that time.
Since nitrate is stable, teachers and students can make water collections that represent wide geographic regions and demonstrate nitrate pollution from a variety of sources. They can even buy and test bottled water from all over the world.
Recently, a new nitrate test kit has been developed by LaMotte Company that uses zinc reduction (eliminating cadmium, a toxic heavy metal) and 2 simple tablets to measure nitrogen as nitrate in ppm. Accurate as long as the chemicals are fresh, it is both safe and easy to use. Consequently, when we rewrote Living in Water in its third edition, we included nitrate as an issue and added 5 new exercises for nitrate, plus additional watershed activities. Nonpoint source and point source nitrate pollution are measured and modeled in the third edition of Living in Water.
Ideally, first have students study nitrate pollution in class. They then identify a nitrate-enriched environment with these test kits (Fig. 3). Encourage them to ask questions about the source of the pollution and the possible solutions. Classes may plan and do projects that reduce nitrate pollution either through direct action, such as planting forest buffer strips along waterways, or through public education, such as encouraging individuals to reduce fertilizer use. The kits can then be used to monitor the results of the student projects over time.
Since each region varies, detailed ideas for class projects should be researched locally. Have your students use the Internet, email, or letters to contact state and local agencies as well as conservation organizations and environmental educators. The Internet may also lead your students to federal agencies such as the EPA, which has information on nitrate pollution.
Nitrate pollution is a large-scale problem in which each of us plays some role. While governments have a major role in solving this problem, individual education and action are essential to improve the situation.
Figs. 2 and 3 are adapted from Living in Water with permission from the National Aquarium in Baltimore.