My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!

Heather Haley
Product Developer
January 2017
In chemistry, a solution is a type of mixture. The composition of a mixture can be variable or uniform throughout. If the composition of a mixture is variable, it is called heterogeneous. If the composition is uniform, it is called homogeneous. The word “solution” is synonymous with a homogeneous mixture.
Solutions are composed of a solvent and one or more solutes. The solvent is the substance present in the greatest amount. All other components are solutes—substances that dissolve in a solvent. The state of matter of solute and solvent determines the type of solution that is formed.
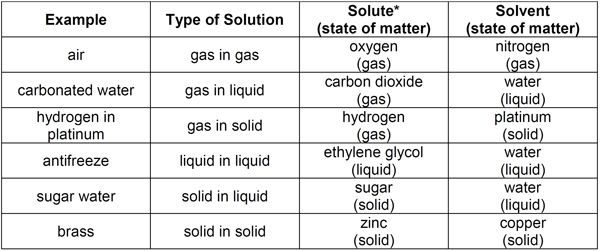
*Some examples contain additional solutes not identified in this table.
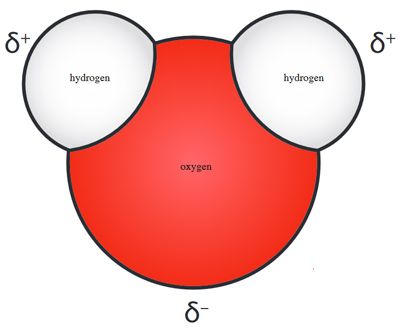
Water is a common solvent used to prepare solutions. By nature of its chemical structure, water is capable of dissolving or dissociating other compounds. Water is a polar molecule: It contains regions of partial positive and negative charge. A positively charged species (atom or ion) will be attracted to the negatively charged end of a water molecule, whereas a negatively charged species will be attracted to the positively charged end of a water molecule. As water molecules move apart, the compound will dissolve or dissociate. When a substance is dissolved in water, an aqueous solution is formed.
The overall composition of a solution can be described both qualitatively and quantitatively. Qualitatively, dilute solutions contain very little solute, whereas concentrated solutions contain a lot of solute. There are many different methods for describing the composition of a solution quantitatively: molarity, normality, percent by mass, and percent by volume. These techniques are fully described in Carolina’s Solution Preparation Manual.