My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
By Mike Isley
Product Developer
We innately explore, observe, question, and experiment to understand our environment. Children demonstrate this propensity at an early age by asking endless “why” and “how” questions—the genesis of a systematic process of thought and investigation referred to as the scientific method. In this simple activity, your students will learn the steps of the scientific method as they investigate the endothermic reaction of effervescent tablets dissolving in water.
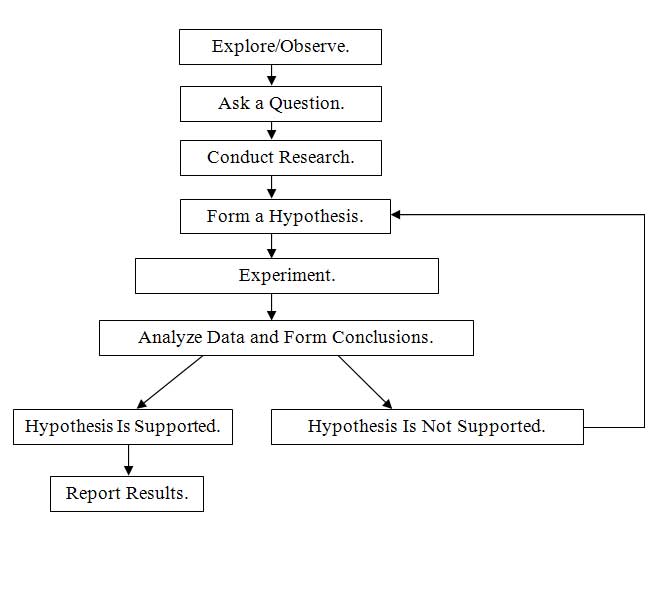
The steps of the scientific method are outlined in a flowchart below. Note the pathway for revisiting the hypothesis and creating a new one if the first one is not supported by the experiment.
Physical Science
Grades 5–8
Grades 9–12
Science as Inquiry
Grades 5–12
Use safety glasses or goggles when conducting this investigation.
Each student group of 2 to 4 needs the following:
Guide your students in doing the following activities.
Why did the temperature of the water decrease when an effervescent tablet was added?
If you were to do research, you would find that dissolving certain substances in water may be exothermic (releasing heat) and certain others, endothermic (absorbing heat).
Because the temperature of water drops after an effervescent tablet dissolves in it, the reaction must be endothermic. Hypothesis: I think that increasing the number of tablets added to a series of identical 50-mL samples of water (from the same source and maintained at the same temperature) will decrease the temperature of each solution by an amount proportional to the number of tablets added.
Following the process you used with 1 tablet in the first container, you will add tablets to a container, stir while measuring the temperature until it remains constant, and then record the reading.
| Number of Tablets | Temperature (°C) |
| 0 | 23.0 |
| 1 | 20.2 |
| 2 | 18.0 |
| 3 | 16.0 |
| 4 | 14.0 |
Figure 2 Sample data table.
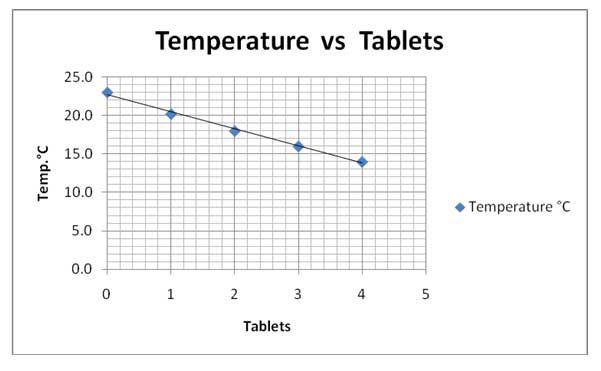
Figure 3 Sample graph: water temperature vs. number of tablets.
The graph of the data supports the hypothesis. The data confirm direct relationship between the increase in number of tablets and the decrease in temperature.
Have the student groups compare their data as a class. Are there any significant discrepancies? If so, discuss possible reasons.