My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
Daniel J. C. Herr, Professor and Nanoscience Department Chair
The Joint School of Nanoscience and Nanoengineering
Greensboro, NC
Nanotechnology is concerned with development and utilization of structures and devices with organizational features at the intermediate scale between individual molecules and about 100 nm (nanometers), where novel properties occur as compared to bulk materials. It implies the capability to build up tailored nanostructures and devices for given functions by control at the atomic and molecular levels. Nanotechnology is recognized as an emerging enabling technology for the 21st century, in addition to the already established areas of information technology and biotechnology. This is because of the scientific convergence of physics, chemistry, biology, materials, and engineering at nanoscale, and of the importance of the control of matter at nanoscale on almost all technologies.1 Nanotechnology is not just a new field of science and engineering, but a new way of looking at and studying materials.

|
Figure 1 A gold ring. |
As one looks at ever smaller scales of the materials and resources around us, new properties and application opportunities emerge. The following example represents 1 aspect of how extremely small dimensions of well-known materials impact their behavior.
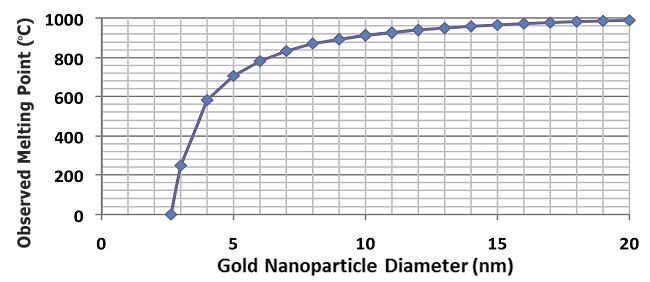
Many bulk metals typically melt at temperatures above 1,000° C. For example, the melting point of gold, such as that for the ring shown in Fig. 1, is 1,064° C (1,947.97° F). Is this melting point a constant? Does a solid material's melting point always occur at the same temperature? Let's consider what happens to the melting point of increasingly smaller pieces of gold. If one removed a sand-sized piece of gold—1 mm in diameter—from that ring, it would melt at the expected melting point of 1,064° C. A particle of gold the size of a red blood cell at 1 µm—which is about 1,000 times smaller than a piece of sand—would melt very near 1,064° C. However, as gold particles approach the size of a protein—10 nm, which is about 100 times smaller than a red blood cell—the observed melting point begins to decrease rapidly with particle size. In fact, a 1-nm gold nanoparticle would become a liquid at room temperature, 20° C. The observed trend in melting temperature, as a function of the size of the gold nanoparticle, is shown in Fig. 2.
To put this into dimensional perspective, if the diameter of a 1-nm particle covered the length of a 1" paper clip, then the ring shown in Figure 1 would stretch from Los Angeles to San Francisco, with a diameter of about 650 km. In other words, a person living in the nanoworld would look at us the way we would look at a person whose body would be as long as 7 planets the size of Jupiter, stacked end to end, or about 1 million km.
|
Figure 2 The impact of particle size on the melting point of gold.2 |
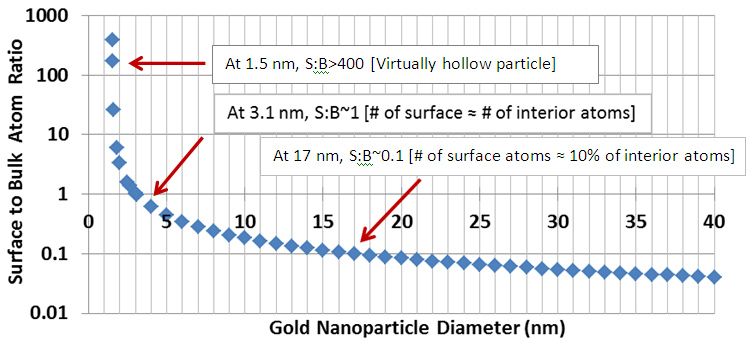
The ring shown in Fig. 1 contains more than a million gold atoms on the inside of the structure for every gold atom on its surface. Let's call any atom on the inside of a material a bulk atom (B), which allows them to be differentiated from the atoms that coat the surface (S) of a material. Fig. 3 shows a plot of the surface to bulk (S:B) atom ratio for various diameter gold nanoparticles, with diameters less than 40 nm. For 17-nm gold particles, the relative number of surface atoms approaches 10% of the bulk atoms within the nanoparticle. Similarly, for 3.1-nm gold particles the S:B is about 1, which means that the number of gold atoms on the surface roughly equals the bulk gold atoms inside the nanoparticle—each numbering around 460. A gold nanoparticle below 1.5 nm in diameter may be like a hollow ball, with very few stable bulk atoms in its interior.
|
Figure 3 Plot of surface to bulk atom ratio as a function of gold nanoparticle diameter. |
This surface-to-bulk atomic volume ratio is a significant contributing factor to the melting point depression observed in gold and other pure metals.2,3 For example, the melting points of 4- and 3-nm gold particles are 581° C and 248° C, respectively, as shown in Fig. 2. Correspondingly, their S:B ratios increase from approximately 0.63 to 1.06, respectively, which suggests that the melting point of gold nanoparticles varies inversely with the particles’ S:B ratio. It is noteworthy that the surface atoms are not completely surrounded by other atoms, but present exposed and unbound surfaces. Consequently, it is reasonable to expect that exposed atoms would exhibit different behavior and reactivity than the embedded bulk atoms. This trend can be extended to other metallic systems, such as copper. Copper nanoparticles with diameters in the range of 3 to 5 nm will melt at about 130° C.4
Melting point depression is but 1 property of nanoscaled materials. For some other useful “nanoworld” opportunities, please consider Professor Richard Feynman's 1959 presentation to the American Physical Society at the California Institute of Technology entitled, "There's Plenty of Room at the Bottom."5 Feynman's presentation was a pivotal moment in the evolution of the field of nanoscience, and a work that every person interested in this field should read.
"The real voyage of discovery consists not in seeking new landscapes but in having new eyes."
—Marcel Proust