My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
 Brent Atkinson
Brent Atkinson
Product Developer
Fall is a beautiful time of year. Nights start to get cooler, the air seems crisp, and Mother Nature puts on 1 of her most beautiful shows. People drive for hundreds of miles to see the bright reds, purples, oranges, and yellows that seem to appear in trees overnight. Fall color tours have become a billion dollar industry in the United States alone. The USDA runs a telephone hotline each fall to alert travelers to the best times to visit various regions. We know the colors have the ability to slow traffic and turn heads, but where do they come from?
The short answer is that they have been hiding in plain sight.
The majority of leaves appear green to the human eye simply because the chlorophyll that fills them up cannot easily absorb green light to be used for photosynthesis. They are better able to use light in the blue and red wavelengths. When the light from the sun hits the leaves, the green light is reflected. The reflected green light travels to our eye and our brain perceives that the leaves are green.
Plants continue to make chlorophyll throughout the summer as long as they can receive enough energy from the sun in the form of light to produce sugar. Chlorophyll is easily decomposed by sunlight so during the spring and summer months, leaves are constantly producing new chlorophyll to replace old chlorophyll, similar to how our bodies are constantly producing new skin cells to replace the old cells. As fall approaches, the number of hours of daylight decreases and the plants stop producing chlorophyll. The chlorophyll remaining in the leaves starts to decompose but is not replaced. When this happens, other pigments in the leaf (that are more stable than chlorophyll) become visible. These pigments were already present in the leaves, but because the eye was being inundated with so much green light, they could not be detected. These pigments include 2 carotenoids: carotene and xanthophyll.
Carotenoids, such as beta carotene, absorb light in the blue spectrum. They do not absorb longer wavelengths well, and they provide us with the bright yellows and oranges in the fall color palette. Carotene is responsible for the orange hues the eye detects. It is the same pigment found in carrots that gives them their characteristic orange appearance. Xanthophyll is responsible for the yellow hues seen in leaves along with flowers and vegetables. Xanthophyll is the pigment responsible for the yellow appearance of a yolk in an egg. These pigments are in the leaves to help capture sunlight and convert it into chemical energy.




Figure 1 A variety of leaves showing the characteristic yellows and oranges from xanthophyll and carotene.
What about the brilliant red color many leaves exhibit during their fall color show? Anthocyanin, a pigment theorized to be protective to the tree or plant, is responsible. Anthocyanins, unlike carotenoids, are not necessarily present in the leaves all season. They are thought to be formed by the leaves to protect them from excessive sunlight and to recover nutrients before the leaves fall to the ground. This helps explain why reds may be vibrant in one geographic area and absent in another. Anthocyanins absorb light in the blue-green spectrum and reflect red. They are responsible for the red colors in oaks and maples, along with other species. Anthocyanins are produced by a reaction between proteins and sugars in the sap. The higher the sugar content of the sap, the brighter the red color. When the nights get longer and cooler, the tree forms an abscission layer (a layer of cells that blocks the transport routes from the leaf to the plant) at the stem of the leaf, trapping any remaining sugars. As the chlorophyll is decomposing, more anthocyanins are being produced. The color of anthocyanin is also dependent on the pH of the tree’s sap. More acidic sap will produce brighter reds while less acidic sap will produce deeper purple-reds. Anthocyanins can even appear blue under certain conditions.



Figure 2 A variety of leaves showing the characteristic reds from anthocyanins.
Eventually, almost all of the pigments break down since they have been cut off from the tree by the abscission layer and leaf color begins to fade. The only pigment still remaining in the leaves is tannin, which gives them their final color: brown.
While every deciduous leaf goes through this process, the vibrancy of fall colors is dependent on a few variables such as temperature, hours of sunlight, and annual rainfall. You may have noticed New England, Wisconsin, Michigan, and other northeastern states are better known for the chromatic display nature puts on each fall. These regions provide everything needed to promote the process of color change. There is ample sunlight all summer long, and most years provide plenty of rain. As fall approaches, the days quickly shorten, and the nights quickly cool. This triggers the tree to ramp down chlorophyll production and start its yearly display of colors.


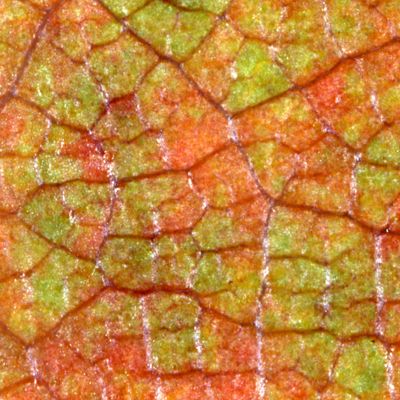

Figure 3