My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!

Dee Dee Whitaker
Product Content Specialist
March 2017
Having good math skills is important and necessary for students to be successful in physical science courses. As a course progresses, formulae build up, units become more complex, and students may become confused. This Physical Science Math Review can help students organize formulae by topic and recall the appropriate SI units; it also gives them a metric conversion and scientific notation refresher. Use the math review from day one or as an end-of-course exam review. Math is the language of science.
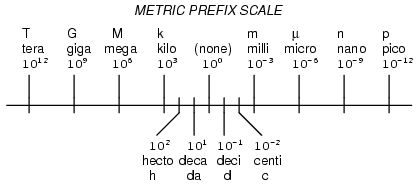
Conversion factor method:
Given quantity × conversion factor = desired unit
Given quantity × desired unit = desired unit
given unit
Example:
Convert 450 mL to L.
![]()
Metric prefix exponent method:
(Exponent of given unit) – (exponent of desired unit) = number of places and direction to move decimal point
Negative answer: move the decimal to the left
Positive answer: move the answer to the right
Example:
Convert 450 mL to L.
milli = –3 Liter = 0
(–3) – (0) = –3
Move the decimal point 3 places to the left from its original position: 0.450 L
Scientific notation converts a large or small number into a number with a mantissa between 1 and 10 multiplied by 10 raised to an exponent.
Steps:
Example:
Place 234,000 into scientific notation.
The decimal is behind the last 0 and must be moved 5 places to the left.
The answer is 2.34 × 105.
Problem-solving steps:
Example:
If a sample of a substance has a density of 2.1 g/mL and a mass of 8.0 g, what is its volume?
Given: D = 2.1 g/mL m = 8.0 g
Unknown = volume mL
D = m/V so V = m/D
![]()


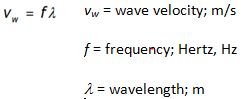
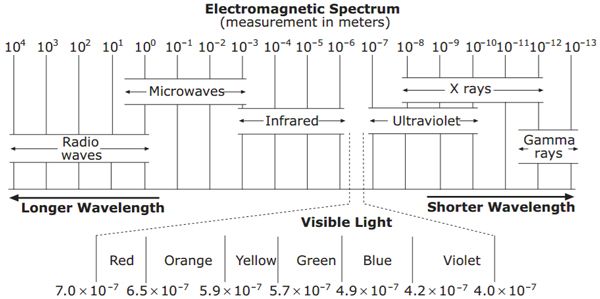

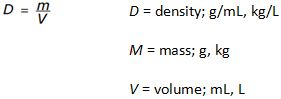
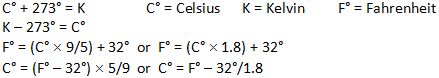
Specific Heat in J/gC°
Summary of Radiation Types and Properties
Type of Radiation |
Alpha particle | Beta particle | Gamma ray |
| Symbol | 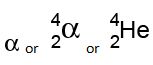 |
|
|
| Mass (atomic mass units) | 4 | 1/2000 | 0 |
| Charge | +2 | –1 | 0 |
| Speed | slow | fast | very fast (speed of light) |
| Ionizing ability | high | medium | 0 |
| Penetrating power | low | medium | high |
| Stopped by | paper | aluminum | lead |
Balancing nuclear equations:
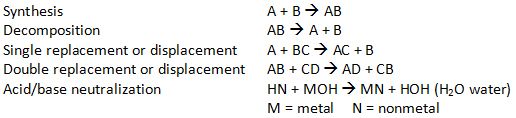
1 mole = 6.02 × 1023 particles particles may include atoms, molecules, ions, electrons
1 mole of a gas at Standard Temperature and Pressure (STP) occupies 22.4 L
Conversion factors: grams/molar mass = moles = particles/6.02 × 1023 particles
pH = –log[H+] or pH = –log[H3O+] [ ] = concentration
pOH = –log[OH-]
pH + pOH =14
Kw = [H+] × [OH-] = 1 × 10-14 at 25°C