My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
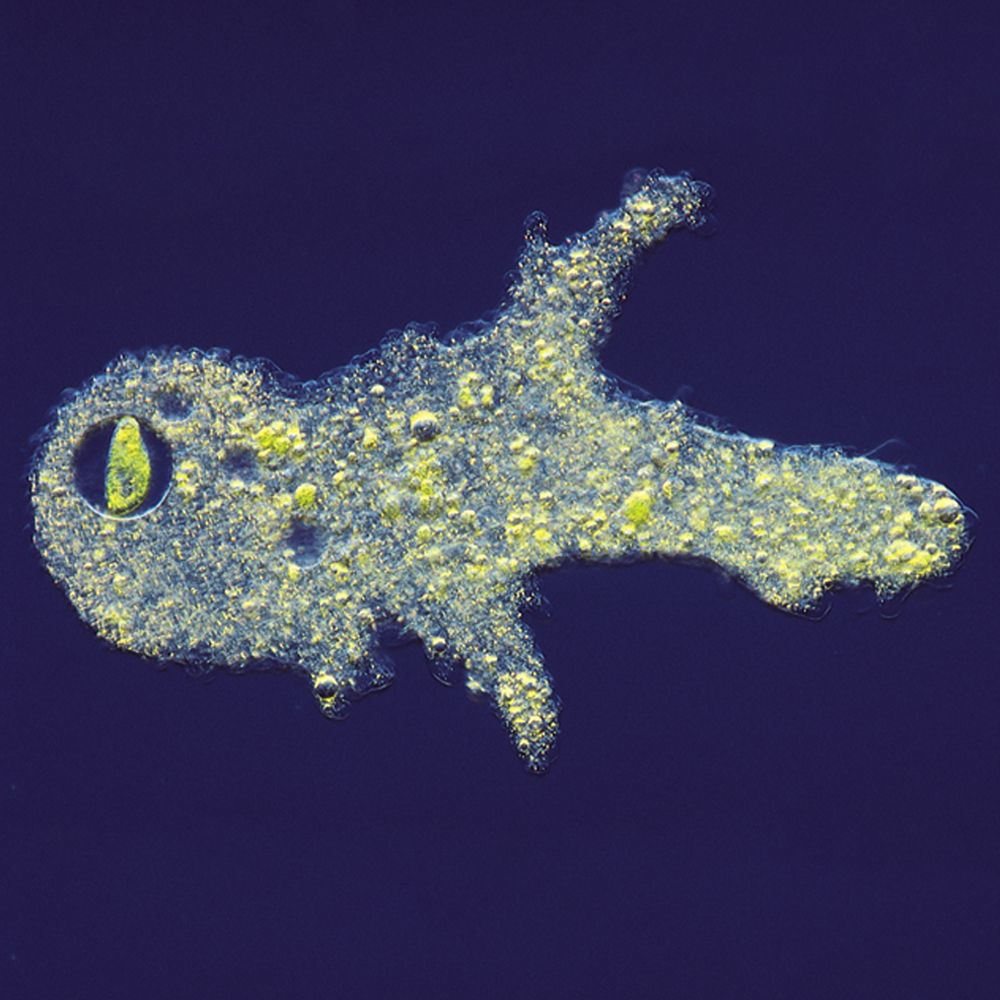
In this lab, students observe a variety of protists and record basic observations of them. Traditionally, protists were classified under Domain Eukarya, Kingdom Protista, and were divided into phyla mainly on the basis of their mode of locomotion. However, it has long been recognized that Protista is not a natural grouping, and that mode of locomotion has little bearing on the evolutionary relationships within these organisms. Today, protist is best used to denote a grade of organization including eukaryotic organisms that are unicellular or multicellular without tissues. Protozoa are protists with some animal-like characteristics, and algae are protists with some plant-like characteristics. Protists are distributed among several clades, many of which are not closely related. Refer to current textbooks and other sources for the most up-to-date classification schemes.
Protozoa Classification Set (131055) for up to 30 students or 60 students working in pairs
This LabSheet can be used with your choice of individual protist cultures or the following Sets:
Easy-View Protozoa Set (131025)
Protozoa Demoslide Set (131006)
Some protists swim so rapidly that it difficult to keep them in the field of view. This is especially true of ciliates, such as Paramecium. These protozoa can be slowed down with Protoslo® (885141). Thoroughly mix a drop of Protoslo with the drops of culture on a slide before adding the coverslip. The Protoslo and culture must be mixed well, or the protists may be forced to the margins when the coverslip is added.
Ensure that students understand and adhere to safe laboratory practices when performing any activity in the classroom or lab. Demonstrate the protocol for correctly using the instruments and materials necessary to complete the activities, and emphasize the importance of proper usage. Use personal protective equipment such as safety glasses or goggles, gloves, and aprons when appropriate. Model proper laboratory safety practices for your students and require them to adhere to all laboratory safety rules. None of the protists included in these sets is parasitic or pathogenic. Even so, know and follow your district’s guidelines so you are prepared if a student should ingest a culture. Cultures remaining after the completion of the activities can be flushed down a sink with tap water. The chlorine and chloramines in most tap water will kill the ptotists. If your tap water is not chlorinated, pipet 1 mL of bleach (sodium hypochlorite) or isopropanol (rubbing alcohol) into the culture and wait 15 minutes before flushing down the sink.
Students can work individually or in groups of 2.
When you receive your cultures, remove them from the shipping container. Remove the lids. Use the dropping pipets included with your order to aerate the culture. Use a separate pipet for each culture. After aerating, replace the lids loosely on the jars but do not screw them down. Leave the cultures undisturbed for 5 to 15 minutes, and then examine them with a dissecting scope (stereomicroscope) at 20 to 40X. Identify areas where protozoa are concentrated. Have students take their samples from these areas.
Set up workstations for each culture. Each station should have the following:
Occasionally a student may make a slide that does not contain any protists. This is usually due to improper sampling technique. If students squirt water from the pipet back into the culture jar or use the pipet to stir the culture, this can disperse the protists and make them harder to find. If the student squeezes the bulb while the pipet tip is in the culture, this can sweep protists away. Some students may have trouble finding an amoeba. This can either be because there is no amoeba on the slide or because the student does not recognize the amoeba. Amoebas are slow moving and can look like a bit of debris on the slide. Some students will find them quickly. Students who are not finding amoebas should look at the slide of a student who has found one. If they still do not find one, they can prepare a new slide.
Failure to handle slides or coverslips by their edges can result in transfer of soap residue to the wet mount. Suspect this if you see sluggish, misshappen, or ruptured protists. If this becomes a problem, demonstrate again the correct way to handle slides and coverslips by their edges. Some students may have to rinse their hands under running water and dry with plain paper towels before continuing.
Optional: Students can be assigned to give currently acceptable classifications for the protists observed. They can also be asked to compare one or more of the protists with plants and animals to discover how they are alike and different. Venn diagrams can be used to make the comparisons. Stained microscope slides show structures not readily apparent with live specimens. Recommended slides are Amoeba proteus (295384), Euglena (295666), and Paramecium (296914). The following slides show pathogenic forms: Entamoeba coli Trophozoites (295450), Trypanosome rhodensiense (295822), Plasmodium falciparum (297190).
The following table is filled in for the protozoa included in our 131020 Protozoa Set, which includes 15 protozoa cultures. Use the data entered here as a guide only. Students may record other data that is equally valid.
Protist |
Unicellular or Colonial |
Shape |
Structure(s) for Movement |
Color |
Specialized Features |
| Actinosphaerium | Unicellular | Spherical or starshaped | Vacuoles for flotation | Gray, clear | Gray, clear |
| Amoeba | Unicellular | Changing | Pseudopods | Gray, clear | Contractile vacuole, granules |
| Arcella | Unicellular | Spherical or flattened sphere | Pseudopods | Brown (test) | Test |
| Blepharisma |
|
Asymmetric oval | Cilia | Pink, pale rose | Contractile vacuoles, oral groove |
| Chlamydomonas | Unicellular | Oval | Flagella | Green | Has chloroplasts; photosynthetic |
| Didinium | Unicellular | Oval, barrel-shaped | Cilia | Gray, clear | 2 bands of cilia; eats paramecium |
| Difflugia | Unicellular | Oval | Pseudopods | Gray, clear, yellowish | Test with sand grains |
| Dileptus | Unicellular | Elongate, pointed posterior | Cilia | Gray, clear | Proboscis with toxicysts |
| Eudorina | Colonial | Spherical | Flagella | Green | Has chloroplasts; photosynthetic |
| Euglena | Unicellular | Elongate | Flagella | Yellow-green | Has chloroplasts; photosynthetic |
| Euplotes | Unicellular | Oval | Cilia | Gray, clear | Cirri (tufts of cilia) |
| Paramecium |
|
Asymmetric oval | Cilia | Gray, clear | Contractile vacuoles, oral groove |
| Spirostomum | Unicellular | Very long, worm-like | Cilia | Gray, clear | Cilia in rows |
| Stentor | Unicellular | Narrows toward posterior | Cilia | Blue | Cilia around “mouth” and in rows |
| Volvox | Colonial | Spherical | Flagella | green | Has chloroplasts; photosynthetic |