My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
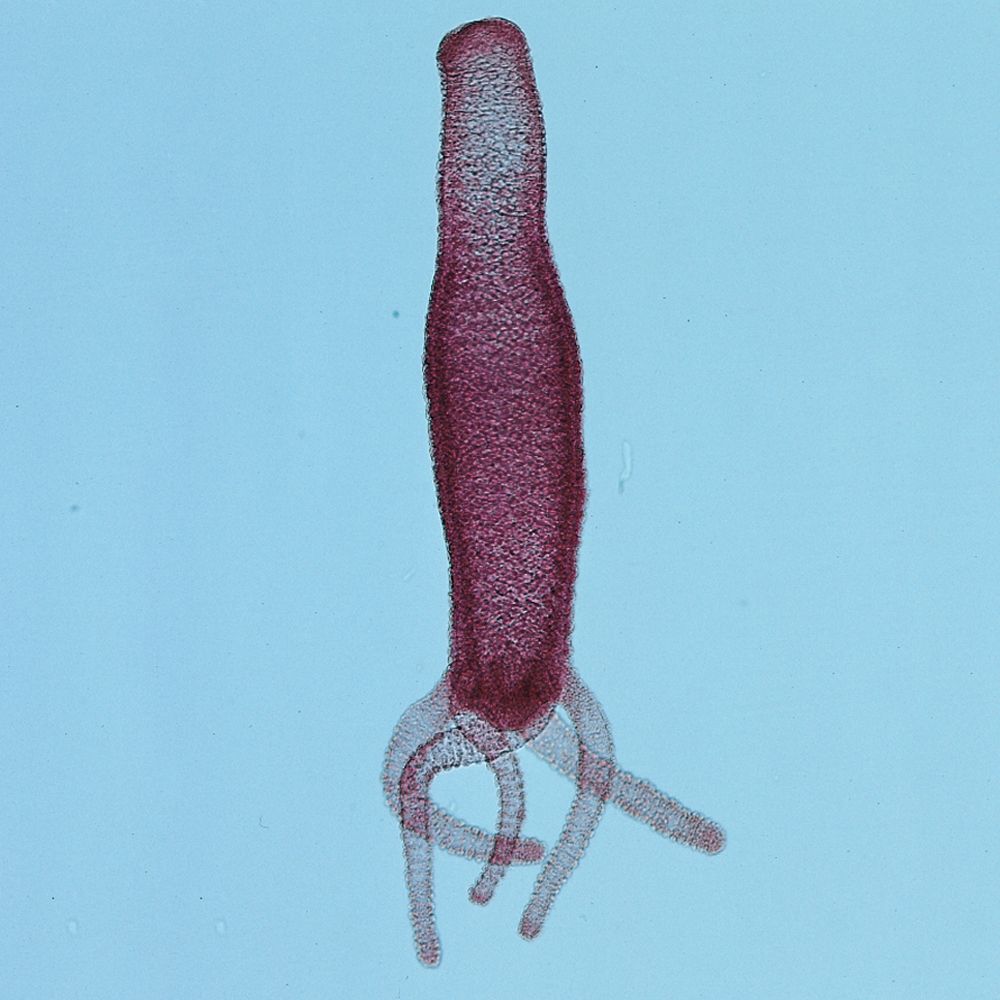
This lab introduces students to Phylum Cnidaria. The activity also serves as an introduction to animals.
Hydra (132800, for 30 students)
Daphnia pulex (142314) or freshly hatched brine shrimp
razor-sharp scalpels (625980), single-edged razor blades (626931), or utility knifes
Bibulous Paper (634050) or paper towel
0.1 M acetic acid, or 1 volume of household vinegar diluted in 5 volumes of water
You may substitute Deep-Well Slides (603730E) for the concavity slides. Because of their greater capacity, however, you would need to transfer several Daphnia to a Deep-Well Slide in order to have a good chance to observe feeding (or, use a pipet to suction enough water out to restrict the movement of a single Daphnia). If stereomicroscopes are not available, hand lenses will suffice. If your compound scopes are equipped with 4x scanning objective lenses, the 40x option may also substitute for stereomicroscopes. You may make copies of the Hydrozoa Anatomy sheet for reference when students are observing the stained longitudinal section and identifying structures.
Ensure that students understand and adhere to safe laboratory practices when performing any activity in the classroom or lab. Demonstrate the protocol for correctly using the instruments and materials necessary to complete the activities, and emphasize the importance of proper usage. Use personal protective equipment such as safety glasses or goggles, gloves, and aprons when appropriate. Model proper laboratory safety practices for your students and require them to adhere to all laboratory safety rules. Caution students about the use of the sharp scalpels or razor blades and supervise their use. Cultures remaining after the completion of the activities can be added to a classroom aquarium or disposed of by flushing down a sink with tap water. The chlorine and chloramine in most tap water will kill Hydra. If your tap water is not chlorinated, pipet 1 mL of household bleach (sodium hypochlorite solution) or isopropanol (rubbing alcohol) into the culture and wait 15 minutes before flushing down the sink.
If you are using brine shrimp instead of Daphnia for the feeding activity, set up the brine shrimp eggs for hatching at least 24 hours before the lab. See our Brine Shrimp Care Guide for instructions on hatching. The brine shrimp larvae must be rinsed in running tap water to remove the salt and then be transferred to springwater before feeding to hydra. Make the transfer immediately before the feeding activity; brine shrimp larvae survive only about 30 minutes in freshwater.
Do not feed Hydra for at least 4 days before the lab, in order to increase the likelihood that students will observe them feeding. Allowing for 2 days in transit, delay an additional 2 or more days after receipt of the culture before conducting the feeding activity. Although Daphnia are traditionally used for feeding Hydra, any small crustacean can be used. Use Daphnia pulex, not Daphnia magna, which are too large for the Hydra to ingest.
It may not be possible to complete all of the Observing a Living Hydra activity in one lab period. A convenient stopping point is after Step 5, the feeding activity. Students can pick up at Step 6 for the next lab. Even if the Observing a Living Hydra activity is completed in one lab period, it is best to use a fresh Hydra for Step 6. A Hydra that has just captured prey will have discharged many of its nematocysts.
Students can work individually or in pairs.
Set up the following workstations:
| For pickup of Hydra | For pickup of Daphnia | For observing discharge of cnidocytes |
| Hydra culture | Daphnia culture | acetic acid |
| dropping pipet | dropping pipet | dropping pipet |
| concavity slide | scalpels or razor blades (use caution) | |
| bibulous paper |
With green Hydra (132810), students will observe symbiotic green algae within the Hydra’s tissues. Place a Hydra in a drop of water on a slide. Add a coverslip to compress it and observe under low and high power. To release algae cells for better viewing, cut off a portion of a tentacle and place it in a drop of water on a slide. Add a coverslip and apply pressure with the eraser of a pencil to crush the tentacle. This will release the algal cells into the water.
Students can design their own experiments to test the response of Hydra to other stimuli. Possibilities include responses to pH, gravity, salt concentration, and light.