My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
 Adrian Dingle
Adrian Dingle
Chemistry Teacher and Author
http://www.adriandingleschemistrypages.com
Carolina Staff
Updated March 2019
If you're reading this, you're probably an AP® Chemistry teacher who has survived a semester of the curriculum. Congratulations! Perhaps you had a to-do list that looked something like this:
So some things may not have gone entirely smoothly, but how can you ease your burden going forward? Well, in addition to knowing the content that is still ahead, you're also aware of the content that isn't in your future. There are 22 exclusion statements that appear in the course and exam description that are designed to tell you what's "out."
Here's a quick guide to the stuff you can omit. These exclusion statements are found in the appropriate Essential Knowledge sections of the AP® Chemistry Course and Exam Description, available for download at AP Central. Note: These comments are my own interpretations and come with the disclaimer that they could significantly change over time, such as when more exam questions and knowledge about the course come to light.

Math is not my strong point. Frankly, that puts me in good company with a number of AP® Chemistry students. For those kids, a lack of math acumen can undermine what could otherwise be some very good progress in chemistry. What can we do to prevent that shortcoming from damaging the scores of those students?
When I was an undergraduate, I took a course entitled Math for Chemists. It was for those of us who were strictly "chemists" but in need of some targeted pointers that might help us to overcome some of the mathematical challenges associated with physical chemistry. Math for Chemists helped me to navigate some scary moments amidst wave functions, the Schrödinger equation, nasty derivatives, and Hamiltonian operators. Ever since that experience, I've always felt that some similar, stealthy tips, albeit at a much lower level, have the potential to be really quite useful for the chemistry student who, like me, will perhaps never learn to love mathematics.
Don't be alarmed
I'm not about to enter into a discussion about the mathematical niceties of quantum mechanics or integrals here, but what I will do is offer math pointers that even I can give to students if they are struggling with a particular quantitative aspect. The list is not an effort to teach math by any stretch of the imagination, nor will it necessarily lead to any kind of enhanced mathematical understanding, but it does represent a list of mental shortcuts that just might unlock some points for a few students on the exam.
Nothing here is groundbreaking or perhaps things that you haven't read in the appendix of a chemistry textbook, but a gentle reminder never hurt; and you may be surprised by how reinforcing a few of these simple relationships can help students save chemistry points on the exam. Beyond that, and in terms of multiple-choice questions, some of these tips remain invaluable since the students are bereft of a calculator, and estimation and mental arithmetic remain crucial skills on that part of the exam.
This list is for students—I know that you know this stuff. Some of it falls under the heading of general math tips, and some is more specifically related to chemistry, but all should prove useful in the areas suggested, and perhaps beyond.
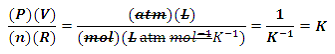
Some may say that, because AP® Chemistry has arguably moved away from the more quantitative aspects of the past, these tips are perhaps less important than they once were. I see it differently.
First, not all of the math has gone away. Logs, exponents, and the ability to estimate are still very relevant. Second, by continuing to use some of the old, quantitative relationships that have actually been removed from the Equations and Constants sheet, one can actually aid the understanding of concepts that have shifted entirely to a qualitative treatment.
Two such examples are root mean square speed and Graham's law of effusion and diffusion. These equations are no longer given with the exam and this means that a quantitative treatment of them is not something that we should expect to see in future exam questions, but qualitative aspects of them are definitely still in play. What does that mean for these 2 examples? Well, not much more than knowing that with a greater molar mass, urms decreases; with increasing temperature, urms increases; and that heavier particles tend to effuse and diffuse more slowly than lighter ones.
The argument that lab work helps to illustrate theory extends and evolves into math work can help to illustrate theory. It's all well and good saying that heavier particles move more slowly, but (even with what some might criticize as being no more than mindless plugging and chugging) we can aid that understanding and cement it with some calculations. Plug the molar masses of 2 different gases into either of those mathematical formulas, pick up a calculator, and you'll see that the resultant numbers bear out what the theory says. For that reason alone, I'll continue to use some of the depreciated quantitative equations in my classes.
 You've overcome the trials and tribulations of the Big Ideas, the Enduring Understandings, the Essential Knowledge, and the Learning Objectives. You pressed on through inquiry labs, particulate diagrams, and Coulombic forces. Depleted by an unusually large number of snow days, you're perhaps scrambling to tie up some loose ends since it's likely been quite a tumultuous year, even for the most experienced. So how can we take stock, remain calm, and set the kids up for exam success?
You've overcome the trials and tribulations of the Big Ideas, the Enduring Understandings, the Essential Knowledge, and the Learning Objectives. You pressed on through inquiry labs, particulate diagrams, and Coulombic forces. Depleted by an unusually large number of snow days, you're perhaps scrambling to tie up some loose ends since it's likely been quite a tumultuous year, even for the most experienced. So how can we take stock, remain calm, and set the kids up for exam success?
For many years, I have offered my kids a last-minute list of "tiny tips" that I think could serve them well going into the exam. A few of the tips are the type of random factoids that occasionally appeared on multiple-choice exams of the past, where perhaps a color or an environmental application was tested for a single point. How relevant that kind of thing will be going forward remains to be seen, but others on the list are reminders of fundamentally important concepts.
As you soldier on through, remember that Carolina has a full line of kits, Carolina Investigations® for AP® Chemistry, specifically designed for the curriculum. The 16 kits in this series address the 6 Big Ideas of chemistry and meet the requirements of the lab curriculum. Each kit focuses on a single Big Idea and offers the option to do either a guided activity or an inquiry activity with your students. To help students prepare for the exam, all kits include Big Idea assessment questions that follow the exam's free-response format.
The College Board's AP® Chemistry lab manual contains 16 inquiry labs that teachers can choose from to fulfill the requirement of 6 inquiry labs in the AP® Chemistry course. The materials and quantities needed for these example labs are listed in the document "Materials for AP Chemistry Guided-Inquiry Experiments: Applying the Science Practices.” The document also includes suggestions for the corresponding Carolina Investigations® for AP® Chemistry kits that meet the same Learning Objectives as the College Board example labs.
*AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, these products.