My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
Adrian Dingle
Chemistry Teacher and Author
http://www.adriandingleschemistrypages.com
Click here to read "Surviving and Thriving in the AP* Chemistry Curriculum, Part 1."
Math is not my strong point. Frankly, that puts me in good company with a number of AP Chemistry students. For those kids, a lack of math acumen can undermine what could otherwise be some very good progress in chemistry. What can we do to prevent that shortcoming from damaging the AP scores of those students?
When I was an undergraduate I took a course entitled Math for Chemists. It was for those of us who were strictly "chemists" but in need of some targeted pointers that might help us to overcome some of the mathematical challenges associated with physical chemistry. Math for Chemists helped me to navigate some scary moments amidst wave functions, the Schrödinger equation, nasty derivatives, and Hamiltonian operators. Ever since that experience, I've always felt that some similar, stealthy tips, albeit at a much lower level, have the potential to be really quite useful for the AP Chemistry student who, like me, will perhaps never learn to love mathematics.
Don't be alarmed, I'm not about to enter into a discussion about the mathematical niceties of quantum mechanics or integrals here, but what I will do is offer my "Top 10" math pointers that even I can give to students if they are struggling with a particular quantitative aspect. The Top 10 is not "teaching math" by any stretch of the imagination, nor will it necessarily lead to any kind of enhanced mathematical understanding, but it does represent a list of mental shortcuts that just might unlock some points for a few students on the AP Chemistry Exam.
Nothing here is groundbreaking or perhaps things that you haven't read in the appendix of a chemistry textbook, but a gentle reminder never hurt; and you may be surprised how, that by reinforcing of a few of these simple relationships, one can go a long way to saving chemistry points on the AP exam. Beyond that, and in terms of multiple-choice questions, some of these tips remain invaluable since the students are bereft of a calculator and estimation and mental arithmetic remain crucial skills on that part of the exam. What's shown below is a list for students—I know that you know this stuff. Some of it falls under the heading of general math tips, and some is more specifically related to AP Chemistry, but all should prove useful in the areas suggested, and perhaps beyond.
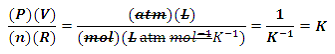
Some may say that, because AP Chemistry has arguably moved away from the more quantitative aspects of the past, these tips are perhaps less important than they once were; I see it differently. Firstly, not all of the math has gone away. Logs, exponents, and the ability to estimate are still very relevant. Secondly, by continuing to use some of the old, quantitative relationships that have actually been removed from the Equations and Constants sheet, one can actually aid the understanding of concepts that have shifted entirely to a qualitative treatment.
Two such examples are root mean square speed and Graham's law of effusion and diffusion. These equations are no longer given with the exam and this means that a quantitative treatment of them is not something that we should expect to see in future exam questions, but qualitative aspects of them are definitely still in play. What does that mean for these 2 examples? Well, not much more than knowing that with a greater molar mass, urms decreases; with increasing temperature, urms increases; and that heavier particles tend to effuse and diffuse more slowly than lighter ones.
The argument that lab work helps to illustrate theory extends and evolves into math work can help to illustrate theory. It's all well and good saying that heavier particles move more slowly, but (even with what some might criticize as being no more than mindless plugging and chugging) we can aid that understanding and cement it with some calculations. Plug the molar masses of 2 different gases into either of those mathematical formulas, pick up a calculator, and you'll see that the resultant numbers bear out what the theory says. For that reason alone I'll continue to use some of the depreciated quantitative equations in my AP classes.
As you soldier on through this year in AP Chemistry, remember that Carolina has a full line of AP Chemistry kits, Carolina Investigations® for AP Chemistry, specifically designed for the AP Chemistry curriculum. The 16 kits in this series address the 6 Big Ideas of chemistry and meet the requirements of the lab curriculum. Each kit focuses on a single Big Idea and offers the option to do either a guided activity or an inquiry activity with your students. To help students prepare for the AP Chemistry Exam, all kits include Big Idea assessment questions that follow the AP Chemistry Exam's free-response format.
The College Board's AP Chemistry lab manual contains 16 inquiry labs that teachers can choose from to fulfill the requirement of 6 inquiry labs in the AP Chemistry course. The materials and quantities needed for these example labs are listed in the document "Materials for AP Chemistry Guided-Inquiry Experiments: Applying the Science Practices". Also included in this document are suggestions for the corresponding Carolina Investigations® for AP Chemistry kits that meet the same Learning Objectives as the College Board example labs.
*AP is a registered trademark of the College Board, which was not involved in the production of, and does not endorse, these products.