My Cart
Your Shopping Cart is currently empty. Use Quick Order or Search to quickly add items to your order!
 Heather Haley
Heather Haley
Product Developer
As discussed in our previous Carolina Tips® article Paper Clip Chemistry, paper clips can be used to teach students about measurement, matter, and atomic structure. Two additional areas of science easily explored with paper clips are chemical reactions and gases.
Show polymerization and different types of polymers by linking paper clips together in a chain. Each paper clip in the chain represents a monomer unit. When all paper clips are the same, the chain is a homopolymer. When more than 1 type of paper clip is used, the chain is a copolymer. There are 3 types of copolymers – alternating, block, and random – and they can be clearly differentiated with colored paper clips. Build a branched polymer using several small chains that are clipped onto 1 long chain. Connecting 2 chains together shows a cross-linked polymer.
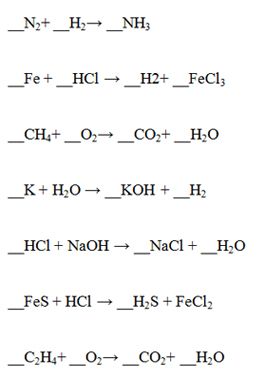
Once students understand how to use colored paper clips to model elements and compounds, they are ready to use them for balancing chemical equations. Construct a model of each element or compound in the products and then in the reactants. Construct additional models as needed to balance the equation. The equation is balanced when both reactants and products contain the same number of each type of paper clip. Keep in mind: If equations are balanced correctly, the mass of reactant paper clips will be equal to the mass of the product paper clips (within an error of 0.1 g).
Suggested reactions for balancing:
Use paper clips and Mylar® balloons to teach buoyancy and the ideal gas law. Attach paper clips to the end to of a helium-filled Mylar® balloon until it is suspended in the air (neither rises nor falls), then remove any string from the balloon. Continue adding paper clips and adhesive notes (or pieces of them) until the balloon remains suspended once again. When the balloon is suspended, its weight is equal to the mass of air it displaces. To find the mass of displaced air,mair, substitute n = m/M into the ideal gas law PV= nRT and solve for m. You may use the current barometric pressure in atmospheres for P, and the current temperature in Kelvin for T. Assume that the balloon is an ellipsoid and calculate its volume using V = 4/3(πa2b) where a is half the balloon diameter and b is half the balloon thickness. The mass of helium in the balloon is found in a similar manner. Remove paper clips and adhesive notes and measure their mass. To find the mass of the deflated Mylar® balloon, subtract the mass of helium from the mass of the displaced air, and then subtract the mass of paper clips and adhesive notes. To find percent error, deflate the balloon and compare its mass to your calculated mass.
